Latest News

Loch Long’s Hawthorn in marathon effort
Loch Long Salmon’s Stewart Hawthorn has run the Boston Marathon, raising funds for an organisation that aims to preserve traditional Gaelic culture. Stirling-based Stewart Hawthorn – Managing Director at Loch Long Salmon – raised over £4,500 for the Gaelic Books...

Mowi ready for a further green bond issue
Salmon farming giant Mowi is set to raise additional finance through issuing more green bonds, the company announced yesterday. In a stock exchange announcement, Mowi said that it has mandated Danske Bank (Green Bond Advisor), DNB Markets, Nordea and SEB...

Arctic Fish escape investigation reopened after appeal
The Icelandic salmon farmer Arctic Fish is likely to face an investigation into its operations in the Westfjords after all. A decision by the police to drop an inquiry into the accidental release of salmon last year has now been...

Gigante confirms new CEO in board shake up
Norwegian land-based salmon farmer Gigante Salmon has a new Chief Executive and has also managed to receive commitment of NOK 120 million (almost £9m) in funding, the company has announced. Gigante Salmon had problems earlier this year, reporting significant mortality...
NEWSLETTER
Stay in touch with the latest developments from Fish Farmer and subscribe to our online updates
What's New
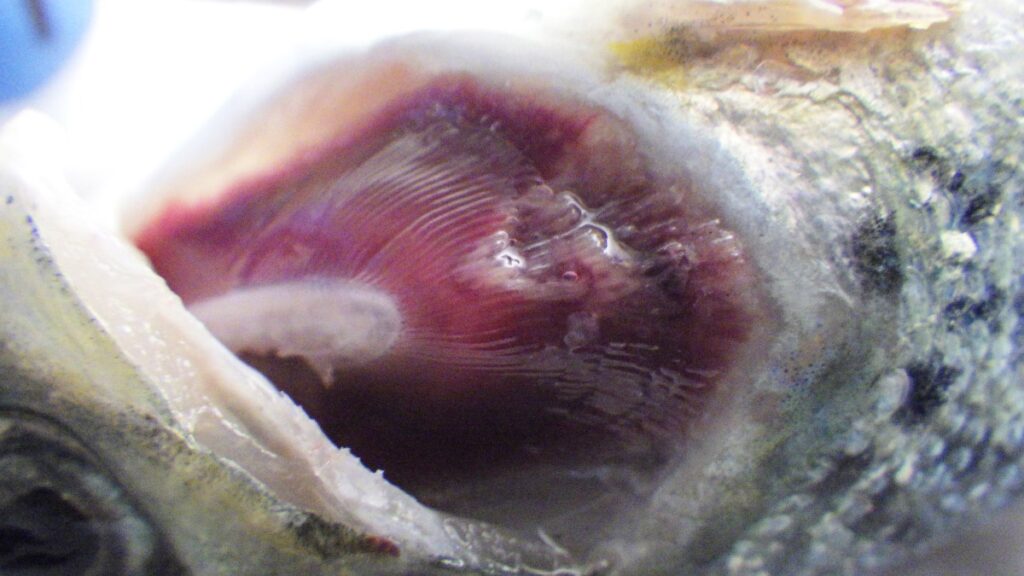
Tenacibaculosis – an alternative approach
The Center for Aquaculture Technologies Canada has launched a tenacibaculosis model to assist farms in combating growing disease challenges In 2023, the Norwegian salmon industry experienced the loss of 62.7...
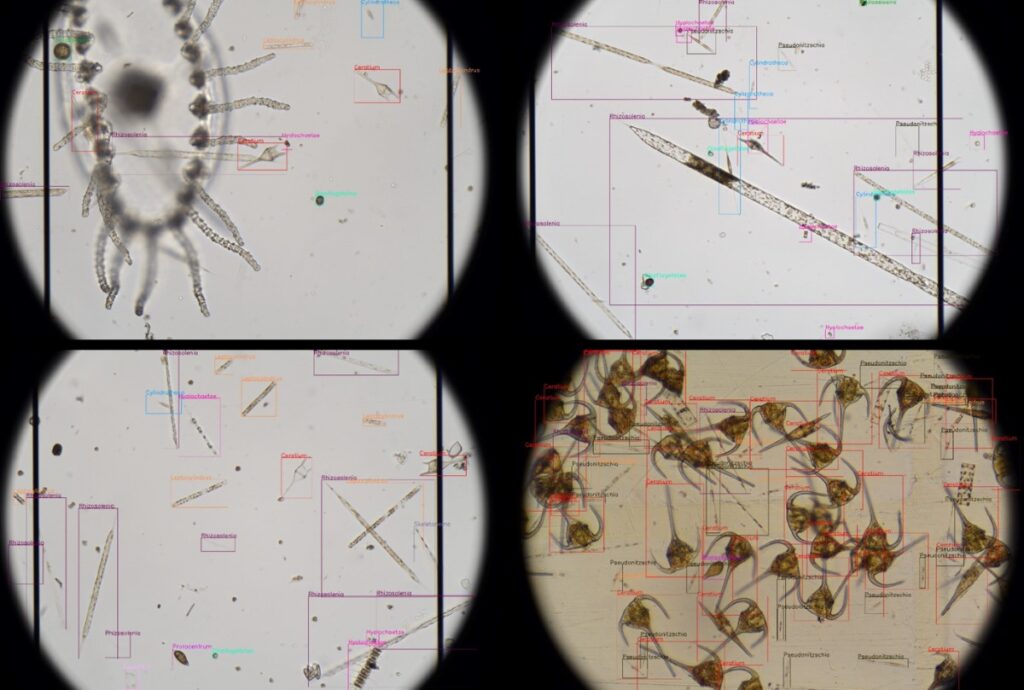
Revolutionising aquaculture safety
OTAQ introduces LPAS to combat harmful algae blooms: a game-changer in aquaculture monitoring Within the aquaculture industry there are increasingly frequent reports of Harmful Algal Blooms (HABS) being responsible for...

BIO-UV and Pinnacle in partnership for ozone treatments
BIO-UV Group has entered into an exclusive partnership agreement with US-headquartered Pinnacle Ozone Solutions to leverage the company’s QUADBLOCK Ozone water treatment products in Europe, Middle East, Africa and Asia. ...

Aquaculture technology and biosecurity
Advanced aquaculture technology is enhancing biosecurity and fish welfare, writes Iain Brown, Industry Sales Engineer, Xylem UK and Ireland Leading aquaculture experts from Xylem will deliver a keynote address at...
Jobs

Marine Engineering Technician, Bakkafrost
At Bakkafrost Scotland we see things from a FRESH perspective. And you can join us. The Marine Engineering Technician will work as directed by the Engineering Manager, across our Marine...
Features

Can you fillet?
Norway’s ban on the export of ‘inferior’ quality salmon is being challenged by the countries who want to process it, reports Vince McDonagh Is Norway heading for a potentially damaging...

Nothing beats getting together in person
Aquaculture industry companies and suppliers are looking forward to the sector’s biggest trade show in the UK As the UK’s leading trade show for the fish farming industry, Aquaculture UK...

Even more at Aviemore
This year’s edition of the UK’s leading aquaculture trade show promises to be bigger and better than ever This May will see the return of Aquaculture UK, which is once...
Opinion

The speed of reaction
Opinion piece by Nick Joy I admit that my mind might not be quite on the job at the moment. Coming back from Sicily by car has been quite an...

Southern exposure
Scotland was well-represented at the Aquasur trade show in Chile, as Mairi Gougeon reports I have recently returned from an official visit to Chile, promoting Scotland’s aquaculture sector and exceptional...

Why we need exports
By Nick Joy Travelling to Europe is a wonderful thing for widening the mind but also reminding me how much we continue to depend on the good taste of our...

Building up an appetite
How to persuade UK consumers to eat more seafood was a key issue at the Norway-UK Seafood Summit, reports Dr Martin Jaffa At the end of February, Norway came to...
Events

Nothing beats getting together in person
Aquaculture industry companies and suppliers are looking forward to the sector’s biggest trade show in the UK As the UK’s leading trade show for the fish farming industry, Aquaculture UK...

Packed programme for Aquaculture UK’s new Innovation Theatre
Aquaculture UK has unveiled the programme for its first ever Innovation Theatre, with presentations running in parallel with existing Keynote Theatre. The trade show and conference will be held over...

Data will drive the fish farm of the future
As technological advancements continue and costs become more accessible, how are traditional and modern fisheries adopting data-driven approaches and intelligent systems into their production processes? This will be one of...
NEWSLETTER
Stay in touch with the latest developments from Fish Farmer and subscribe to our online updates